Medtronic Cardiac Rhythmic Disease Management

Source: Medtronic Website |
Vitatron - G20 SR Diagnose Prevent Treat Rate Drop Response – When syncope is the result of carotid sinus syndrome or vasovagal syncope, the G-series pacemaker recognizes the imminent faint and responds by pacing at an increased rate. This flexible recognition algorithm may help to alleviate symptoms in your patients and prevent their syncope altogether. More information here. |
Source: Medtronic Website |
Sphera SR - SPSR01 Physical characteristics Brief Statement More information here. |

Source: Medtronic Website |
Sphera DR - SPDRL1 Physical characteristics Brief Statement More information here. |
Source: Medtronic Website |
Azure DR - W2DR01 The Azure™ pacemaker is equipped with BlueSync™ technology and is compatible with MyCareLink Heart mobile app — the latest innovation from Medtronic in remote monitoring. If Azure detects changes in your heart, it wirelessly and securely transfers your heart device information to your clinic. Azure pacemaker is safe in the MRI environment when specific conditions are met, and offers exclusive algorithms to accurately detect and reduce the likelihood of atrial fibrillation. Model numbers: W1DR01, W1SR01, W3DR01, W3SR01 More information here. |
Source: Medtronic Website |
Mirro ICD VR Automatic - Simple to Use VT/VF Management Mirro MRI™ ICD is approved for use in the MR environment, when MR conditions for use are met. MRI SureScan™ devices have simple scanning conditions, including: No MRI exclusion zone More information here. |
Source: Medtronic Website |
Mirro ICD DR Automatic - Simple to Use VT/VF Management Mirro MRI™ ICD is approved for use in the MR environment, when MR conditions for use are met. MRI SureScan™ devices have simple scanning conditions, including: No MRI exclusion zone More information here. |
Source: Medtronic Website |
Solara CRTP Personalised CRT + Expanded MRI Access Achieving optimal response can be challenging. Our Solara CRT-Ps offer tools to personalise CRT. These devices are safe for use in the MRI environment when specific conditions are met. The quad model features: MRI SureScan™ Technology The non-quad model features: MRI SureScan™ Technology More information here. |
Source: Medtronic Website |
Serena CRTP - W4TR05 Serena™ CRT-Ps are enabled with BlueSync™ technology, allowing for tablet-based programming and app-based remote monitoring. These devices include an exclusive algorithm to manage atrial fibrillation (AF). PhysioCurve Design for Patient Comfort More information here. |
Source: Medtronic Website |
Percepta CRTP -W4TR04 Percepta™ CRT-Ps are enabled with BlueSync™ technology, allowing for tablet-based programming and app-based remote monitoring. These devices include exclusive algorithms to optimize CRT and manage atrial fibrillation (AF). Enhanced Longevity Physiocurve™ Design for Patient Comfort Exclusive algorithms to manage HF More information here. |
Source: Medtronic Website |
Compia CRT-D MRI The Medtronic Model DTMC1QQ Compia MRI Quad dual chamber, implantable cardioverter defibrillator with cardiac resynchronization therapy (CRT-D) is a multiprogrammable cardiac device that monitors and regulates the patient’s heart rate by providing single or dual chamber, rate-responsive bradycardia pacing; sequential biventricular pacing; and ventricular tachyarrhythmia therapies. The device can detect ventricular tachycardia and ventricular fibrillation (VT/VF) automatically and provide treatment with defibrillation, cardioversion, and antitachycardia pacing therapies. The device can also detect atrial tachycardia and atrial fibrillation (AT/AF) automatically. Simultaneous or sequential biventricular pacing is used to provide patients with cardiac resynchronization therapy. The device responds to bradyarrhythmias by providing bradycardia pacing therapies. The Compia MRI Quad system is indicated for ventricular antitachycardia pacing and ventricular defibrillation for automated treatment of life-threatening ventricular arrhythmias and for providing cardiac resynchronization therapy in heart failure patients on stable, optimal heart failure medical therapy if indicated, and meet any of the following classifications: New York Heart Association (NYHA) Functional Class III or IV and who have a left ventricular ejection fraction ≤35% and a prolonged QRS duration More information here. |

Source: Medtronic Website |
Micra™ VR MC1VR01 Unlike most pacemakers that are placed in the patient's chest with leads running to the heart, Micra is implanted directly into the patient’s heart. Less invasive — Micra is placed in the heart via a vein in the leg, thus no chest incision, scar, or bump that results from conventional pacemakers. Micra Model MC1VR01 is indicated for use in patients who have experienced one or more of the following conditions: symptomatic paroxysmal or permanent high-grade AV block in the presence of AF Rate-responsive pacing is indicated to provide increased heart rate appropriate to increasing levels of activity. More information here. |

Source: Medtronic Website |
Micra™ AV MC1AVR1 AV SYNCHRONY REIMAGINED Dynamic sensing that adjusts pacing based on the mechanical atrial contraction. 11 new algorithms More information here. |
Source: Medtronic Website |
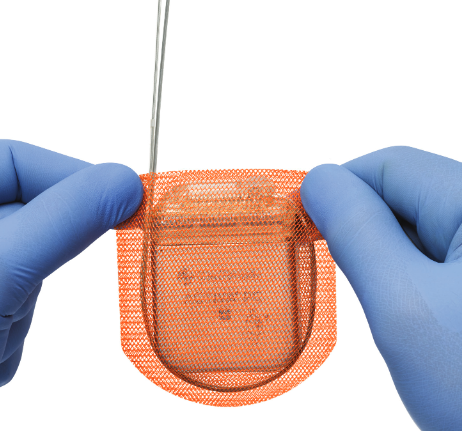
Tyrx - CMRM6133INT TYRX™ The TYRX™ Absorbable Antibacterial Envelope is a fully absorbable sterile prosthesis designed to hold an Implantable Neurostimulator (INS) securely in place to create a stable environment when implanted in the body. The TYRX™ bioabsorbable polymer coating contains antimicrobial agents Minocycline and Rifampicin each in concentrations of 102 ug/cm2 More information here. |

Source: Medtronic Website |
Reveal XT Insertable Cardiac Monitor - 9529 The Medtronic Reveal XT Insertable Cardiac Monitor is a programmable device that continuously monitors a patient’s ECG and other physiological parameters. The Reveal XT records cardiac information in response to automatically detected arrhythmias and patient activation. The Reveal XT is designed to automatically record the occurrence of arrhythmias in a patient. Arrhythmia may be classified as atrial tachyarrhythmia/atrial fibrillation (AT/AF), bradyarrhythmia, asystole, or (fast) ventricular tachyarrhythmia. In addition, the Reveal XT can be activated by the patient to record cardiac rhythm during symptomatic episodes. More information here. |
Source: Medtronic Website |
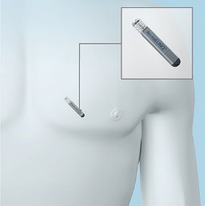
Reveal LINQ - LNQ11 The Medtronic Reveal LINQ ICM is a programmable device that continuously monitors a patient’s ECG and other physiological parameters. The device records cardiac information in response to automatically detected arrhythmias and patient activation. The device is designed to record the occurrence of an arrhythmia in a patient automatically. Arrhythmias may be classified as tachyarrhythmia, bradyarrhythmia, pause, atrial tachyarrhythmia, or atrial fibrillation. In addition, while experiencing or immediately after a symptomatic event, the patient can activate the recording of cardiac information in the ICM. Discreet – The Reveal LINQ ICM is not visible in most patients. More information here. |
Source: Medtronic Website |
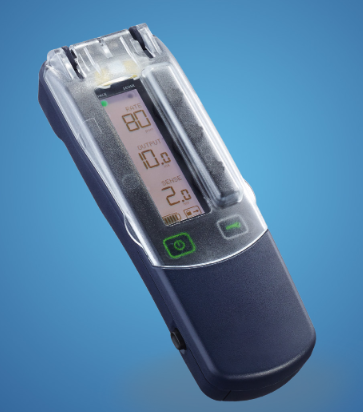
Single Chamber Temporary External Pacemaker - 53401 Introducing temporary external pacemakers Models 53401, our complete EPG portfolio and the next generation of temporary external pacemakers for managing bradycardia. Both Models include easily configurable features, enhanced user interface, and improved low battery indicator. More information here. |

Source: Medtronic Website |
Dual Chamber Temporary External Pacemaker - 5392 Introducing temporary external pacemakers Models 5392, our complete EPG portfolio and the next generation of temporary external pacemakers for managing bradycardia. Both Models include easily configurable features, enhanced user interface, and improved low battery indicator. More information here. |
